APPLIED
SUPERCRITICAL
FLUIDS
About

Romain POLANZ, CEO & Founder of ASF
« With an higher education in « Techniques and Applications of Physics », several experiences in aerospace industry, within the Research Center of Saint-Gobain PAM Group, and within the company Separex – a former world global player of the Supercritical Fluids Technology – as SCF Equipment Designer, Project Leader, then Supercritical Fluids Equipment Division Manager, with more than 150 SCF Units designed, built, qualified and implemented worldwide, I have then been able to gain a solid experience with CBD’s Extraction dedicated to therapeutic purpose at industrial scale, using a Subcritical CO2 Extraction process.
I decided to create ASF company in 2018 in order to promote the Supercritical Fluids Technology as Independent & Expert Consultant: Process Engineering, Dedicated & Tailor Made SCF Equipment, Business Development and Marketing associated to this innovating technology.
Throughout your project, APPLIED SUPERCRITICAL FLUIDS and its partners stand by you and allow you to define, improve and achieve at best your current or future application »
Supercritical Fluids Technology and its many Applications:
What is a Supercritical Fluid?
This state of matter was discovered in 1822 by a French engineer and physicist : Charles Cagniard de Latour
Thereafter, it was used as supercritical fluid term by the Irish chemist Thomas Andrews
Any fluid has a critical point which is reached to specific conditions of pressure and temperature
A fluid is then considered as « Supercritical » thenceforth it is heated to a temperature above its critical temperature and compressed to a pressure greater than its critical pressure
Placed into this Supercritical domain, such fluid has therefore specific physicochemical properties (diffusivity, viscosity, density, thermal conductivity) at the interface between those of a gas and of those of a liquid
One can thus imagine a supercritical fluid as a fluid acting as a gas, while being almost as dense as a liquid, so that it has a dissolving power – as well as a liquid – while having a diffusivity through matter close to that of a gas
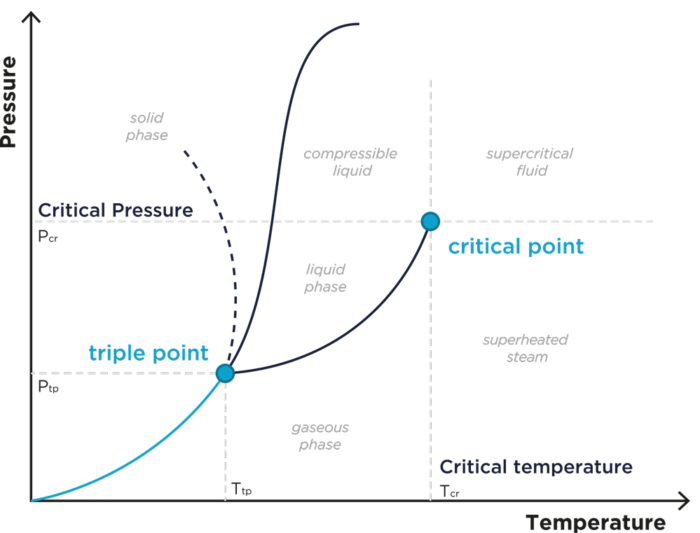

Pressure – Temperature Phase Diagram
What is the interest of using a Supercritical Fluid?
A Supercritical Fluid is typically used for its solvent properties to extract a high value-added compound contained in a solid raw material (vegetable or botanical) such as natural dyes, perfumes & fragrances, aromas, essential oils, antioxidants, active ingredients (API) in the pharmaceutical domain, …
Conversely, the dissolving power of a Supercritical Fluid can be used to remove undesirable compounds, such as pollutants, paraffin / wax, binders used in the preparation of metallic powders or ceramics, TCA’s (trichloroanisoles) that can be found in the cork stoppers responsible for this famous “cork smell” when tasting a wine, …
« Indeed, the dissolving power of a solvent submitted to supercritical conditions considerably increases »
Supercritical Fluids, as well as associated Supercritical Processes, are ultimately always used as alternatives to organic solvents and « conventional » techniques, thus offering the possibility of a “Green” Technology and “Environmentally Friendly”
« The two most commonly used supercritical fluids are the carbon dioxide (CO2) and the water (H2O) »
Supercritical CO2
Carbon dioxide (CO2) is THE worldwide most used supercritical fluid.
Why ?
- Its critical point is relatively low: 31°C and 74 bars, therefore easily achievable
- It is cheap and easily available
- It comes from recycling of industrial waste
- It is considered as a « green » solvent: it is nontoxic, non-flammable, chemically inert and non-polluting in its supercritical phase
- It preserves the integrity of heat-sensitive compounds, with its low critical temperature (31°C)
- It allows selective extraction of molecules, from the same initial raw material
- With a Supercritical CO2 Extraction, final extracts are pure and free from organic solvents
Subcritical and Supercritical Water
As carbon dioxide (CO2) placed in its supercritical area, it is possible to use the solvent properties of water (H2O), which used in its « subcritical » phase allows solubilizing hydrophobic compounds contained within different varieties of plants.
It may be called “superheated water under pressure“, and this extraction process once again allows an alternative to conventional extraction methods, with the use of a “green” solvent, working directly on the initial wet plant material, while improving the selectivity of the molecules to be extracted.
Besides, supercritical water is obtained when submitted to much higher pressure and temperature values (Critical Point: 218 bars and 374°C).
It is interesting to note that, placed in this supercritical zone, properties of water are almost reversed with those of liquid water!
Thus, in this supercritical domain, water becomes a powerful solvent, capable of solubilizing a large number of organic compounds.
On the other hand, it produces a precipitate of all inorganic components, which can seriously and irreversibly damage installations submitted to high pressure and high temperature if they are not properly designed (e.g. Inconel or Titanium liners implemented into high-pressure vessels, to resist penetration of the supercritical water in the metal).
An innovating application thus consists in using water in its supercritical phase to eliminate organic waste or toxic solvents, following an « hydrothermal » treatment
By adding an oxidizer (dioxygen O2 naturally contained in the air, for example) – much more soluble in water in its supercritical phase – the interaction (or “attack”) with organic matter is thus much more powerful and fast.
Compared to traditional organic materials destruction processes (e.g.: Incinerators), there is no formation of « NOx » or « SOx » (Nitrogen Oxides, Sulfur Oxides) and operating temperatures are much lower.
These hydrothermal processes using water in its subcritical or supercritical phases, thus allow to treat liquid or semi-liquid waste without prior processing operation (e.g.: sludges), recycle certain materials (catalysts), certain biomasses valorization, achieve liquefaction purposes, gasification (energy applications), or oxidation


Treatment of ions exchange « resins » beads by Hydrothermal Oxidation
SITEMAP
CONTACT: polanz.romain@appliedsupercriticalfluids.com
18, Rue Henner 54000 Nancy – FRANCE
+33 (0)6.20.48.71.76. / +33 (0)3.54.12.12.78

